- Creating the next-gen therapiesTechnology
What Is Epilepsy?
Epilepsy is a chronic disorder characterized by recurrent and unprovoked seizures. Presently there is no cure for epilepsy, only management of its symptoms. While many respond to current anti-epileptic medications, more than one third of individuals with epilepsy do not and warrant multiple concurrent medications that often exacerbate undesirable side effects. The human brain is the source of epilepsy, although the symptoms of a seizure may affect any part of the body. Some seizures have genetic basis or originate from brain trauma, however, a large number remain “idiopathic” (of unknown cause). While an initial epileptic event may occur in one location in the brain, how it spreads and how much more of the brain is affected determines what effects seizures have on both brain and body functions. As such, these factors determine the clinical definition of the type of epilepsy, the character of the seizure and its impact on the individual. For this reason, classifications of epilepsy are numerous, with varied symptoms making drug development challenging.
It is generally recognized that there have been “three generations” of AEDs developed dating as far back as the early 1900’s and representing some 30 therapeutics although only a handful are commonly prescribed today. Despite these, more than one third of patients are refractory or develop resistance to their medications.
For more information about epilepsy please look to the following websites:
http://www.epilepsy.ca/
https://www.epilepsy.com/
https://www.ninds.nih.gov/Disorders/All-Disorders/Epilepsy-Information-Page
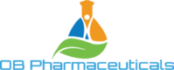
Our Drug Pipeline
OB Pharmaceuticals has developed a new class of compounds that act at a unique target on neurons in the brain to prevent the aberrant electrical activity that leads to seizures. Our compounds not only stop seizures from occurring in epileptic brains; they have shown evidence in our preclinical models to prevent epilepsy from developing (“anti-epileptogenic”). These activities, combined with no observed toxicity or side effects, makes our novel molecules one of the most promising new preclinical therapeutics currently under development. As such we believe they represent the “fourth generation” of leading candidates for the management, and hopefully prevention of epilepsy and brain injury related seizures.
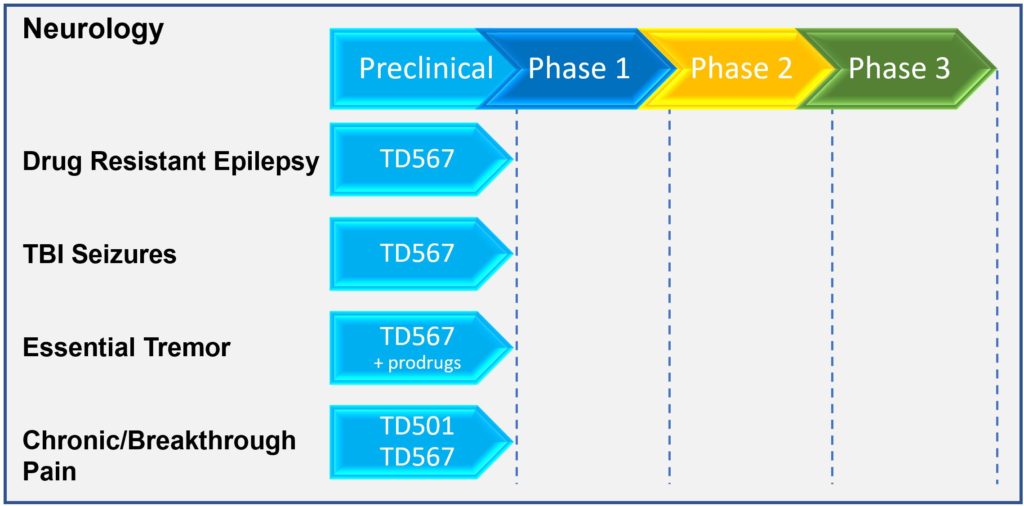
Chemistry and Manufacturing
To date, we have screened more than 100 novel molecules (NCEs) across two chemical families. These molecules were synthesized by our accomplished medicinal chemist, Dr. Tony Durst, Professor Emeritus, Chemistry, at the University of Ottawa, Ontario. Their structures are unlike anything developed thus far or in pipeline development for epilepsy treatment.
Our lead candidate TD567, its prodrugs and analogues are highly stable, easily formulated and consist of low-cost starting materials, scalable synthesis steps, and optimized yield for manufacturing in amounts necessary to meet the needs of any patient population.
Intellectual Property
Patents have been filed for both NCE structural families. The USPTO has granted patents for therapeutic method across both chemical classes: 10,087,138 (2018) and 10,556,853 (2020); and pharmaceutical composition 10,618,870 (2020) for the first. Additional U.S. allowances for pharmaceutical composition are forthcoming. Both class filings have received “green light” PCT reviews and have entered national phases in the 7MM and elsewhere. A third structural class patent filing is under development. All together, we believe that these will constitute a strong intellectual property portfolio.
Drug Screening Process Using Gold Standard and Novel Development Platforms
Our lead screening program consists of two tiers of assays, both conventional (“gold standard”) in the field of drug development for epilepsy and novel, developed in-house, to obtain only those molecules with the most optimal pharmacodynamic (how & where the drug works) and pharmacokinetic (how the body handles the drug) properties. These assays span in vitro and ex vivo (e.g. cells or tissues in culture) and in vivo (e.g. in appropriate animal models) to provide the most robust ability to identify lead candidates with optimal profiles. Examples of the screens are shown below:
VSDI: voltage sensitive dye imaging; DRE: drug resistant epilepsy
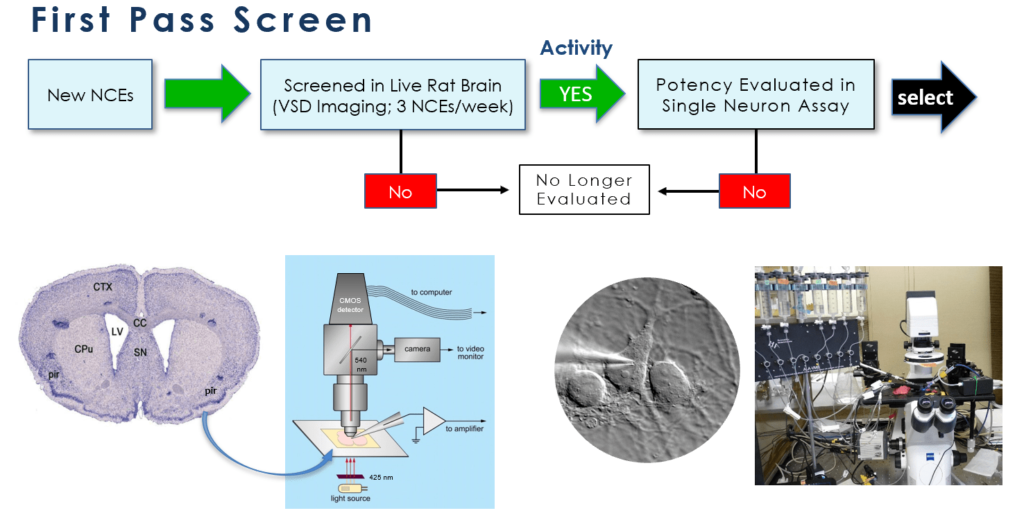
After showing positive results in both neural circuit activation/attenuation assays using voltage-sensitive dye imaging (VSDI) and single neuron electrophysiological assays, promising candidates are then moved to two in vivo animal models of epilepsy development: kindling and seizures induced following traumatic brain injury. The kindling model, one that is similar to complex partial seizures in humans is a “gold standard” assay for AED development. In addition, we employ a unique ex vivo assay of brain tissue that demonstrates drug resistant neural network behaviour. We examine the drug refractoriness of common standard of care AEDs against our lead candidates utilizing frequency modulating VSDI.
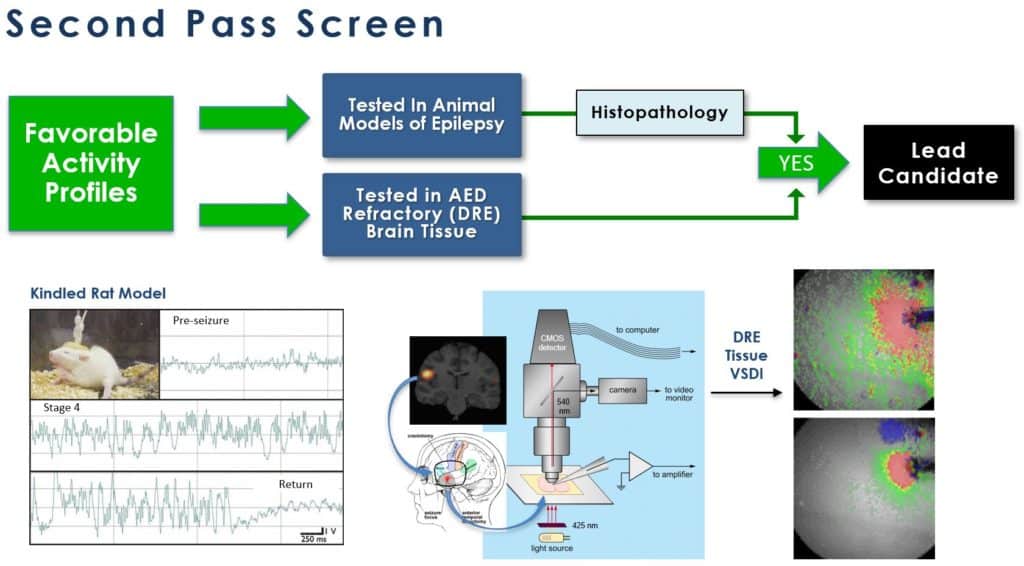
This latter approach, utilizing drug refractory tissue is very important as results obtained to date strongly indicate that our lead candidate (and its prodrug/analogues) could provide effective relief for this inadequately addressed condition. In parallel tests, our compounds are 1,000 times more potent (nM vs. μM) and 4-5 times more effective than commonly used AEDs in attenuating neural network excitation.
A very few lead candidates are then taken through extensive pharmacodynamic and pharmacokinetic analyses along with metabolism, stability, off-target screening, genotoxicity, and general toxicology and side effects studies.
The Epilepsy Therapeutic Screening Program (ETSP) at the National Institute of Neurological Disorders and Stroke (NINDS, NIH)
Since its establishment in 1975, the ETSP has made important contributions to the development of several FDA-approved drugs for epilepsy, including felbamate (Felbatol), topirimate (Topamax), lacosamide (Vimpat), and retigabine (Potiga). AED candidates are accepted once their novelty has been established against the program’s structural database of more than 32,000 compounds. OB Pharmaceuticals was accepted into the ETSP program in 2017 and a limited number of molecules including our current lead TD567 have been progressing through their series of investigational assays. To date, data received support the development of TD567. We expect completion of the full series of assays in 2020.
.
For more information about the ETSP, visit https://www.ninds.nih.gov/Current-Research/Focus-Research/Focus-Epilepsy/ETSP.